“[This] landmark study … should stimulate discussion and prompt improvements in medication safety in the operating room.”
In this month’s issue of Anesthesiology, Nanji and coworkers present a landmark study that should stimulate discussion and prompt improvements in medication safety in the operating room. The authors performed a prospective, observational clinical trial in a tertiary-care teaching hospital, which measured the frequency of medication errors and adverse drug events during the perioperative period. They reported the numbers of errors and adverse events as percentages of the total number of medications administered. In addition, a retrospective chart review was performed to capture medication errors and adverse events that were missed during the observation period. On the basis of their results, the authors developed recommendations that they believe would have prevented the particular errors and adverse events that were observed during the study.
A total of 277 operations were observed, during which a total of 3,671 medications were administered. About 1 in every 20 medications administered involved a drug error and/or an adverse drug event. Specifically, a total of 153 medication errors occurred, about one third of which caused an observable adverse drug event (33.3%). A total of 193 medication errors and/or adverse drug events were observed, of which 153 (79%) were preventable and 40 (21%) were not preventable. No medication-related deaths were observed; however, 133 (68.9%) of the observed or potential adverse drug events were serious and 3 (1.6%) were life-threatening. The single most common type of error was a labeling error (37 events; 24.2%). There was no difference in event rates for patients who underwent general anesthesia (227 cases, 82.0% of the total, 3,297 medications administered, 5.3% event rate) and those who underwent sedation only (37 cases, 13.4% of the total, 374 medications administered, 4.6% event rate). One third of the anesthesia care providers were house staff (n = 93, 33.6%); however, no differences in event rates were observed among house staff (68 events, 5.1% event rate), nurse anesthetists (111 events, 5.5% event rate), and staff anesthesiologists (14 events, 4.5% event rate).
The high error and adverse event rates reported by Nanji and coworkers are surprising and raise several important questions. Why were the rates of medication events substantially higher than those reported in previous studies?
2,
3 Did observers include trivial events or events that simply reflected a difference in opinion (e.g., choice of drug dose)? Despite the uncertainties, several attributes of the study suggest the high event rates are accurate. First, events were detected by direct third-party observation rather than by self-reporting or facilitated incident reporting (e.g., where reports are completed whether a drug error has occurred). The incidence of errors is typically much higher when events are detected by impartial observers rather than through self-reporting or surveys.
4,
5 Second, the observers who detected the events were fully trained, experienced anesthesia care providers, not less experienced research personnel. Third, the authors reported good interrater reliability in the study. These factors could account for the high event rates reported by Nanji and coworkers, compared with previous studies.
For several reasons, medication errors and adverse event rates may be even higher in other care settings such as out-of-hospital or office-based anesthesia care sites and community hospitals. The study was performed at the Massachusetts General Hospital (Boston, Massachusetts), a world-leading medical center in medication safety. Several of the coauthors have published sentinel reports on medication error in hospital inpatients.
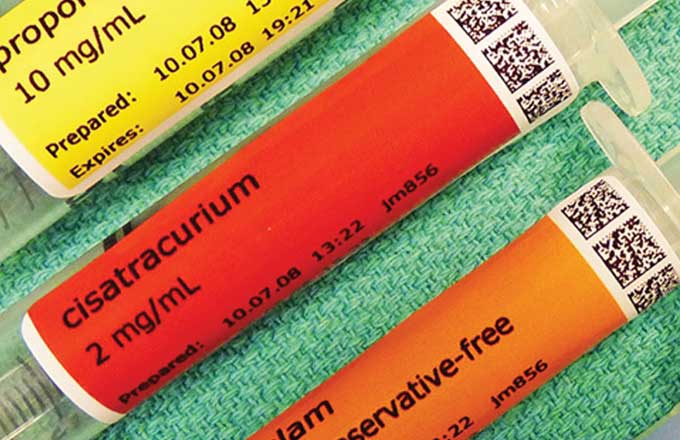
6 This local expertise could heighten awareness about medication safety throughout the institution. In addition, the hospital utilizes a bar code–assisted syringe-labeling system in its operating rooms. The bar-code devices are used to scan medication ampules or vials and print user-applied, color-coded, self-adhesive labels that contain critical drug information. The device also provides audiovisual feedback of the drug name and concentration. The likelihood of errors resulting from mislabeling a syringe or misidentifying an ampule or vial may be higher in institutions that do not use this technology. The hospital also uses an electronic information management system that automatically records anesthesia information into the electronic health record, which may reduce transcription errors. Finally, the anesthesia care providers involved in the study consented to be observed. The act of consent and presence of observers in the operating room could increase vigilance and reduce error rates. Notably, it will be important to independently confirm the event rates reported by Nanji and coworkers in other tertiary-care centers because the criteria used to categorize events were somewhat subjective.
Despite limitations, the best available data indicate that drug errors are not rare, inconsequential events caused by distracted or inexperienced care providers. The results suggest that the first step to reduce errors is to heighten awareness and revise education curriculum regarding drug safety in the operating room. Education is essential but not sufficient to reduce drug errors, as the problem of anesthesia medication safety has been recognized for years. For example, an analysis of closed medical–legal claims against anesthesiologists in Canada for the period of 1998 to 2002 showed that 52% of the adverse events (and 62% of the lasts) resulted from medication-related adverse events.
7 Despite mounting evidence, few interventions have been developed to improve drug safety in anesthetic practice.
The second step is to recognize that the operating room remains one of the last areas in the hospital where “redundancy” or multiple checking, a hallmark of medication safety, is not a common practice. We need to consider adopting new technologies that have been implemented in error-prone environments. For example, bar code scanners allow an electronic double check that could supplement other more error-prone strategies, such as the use of distinct syringe sizes for different medications and placement of different drug items at specific locations on the drug cart. Bar code systems have been successfully implemented in approximately 90% of the hospitals in the United States.
8 In settings outside the operating room, these systems have improved medication safety.
9 For example, in the emergency department, bar code–assisted administration systems reduced the incidence of medication errors by 80.7%.
10 In hospitalized patients, bar code systems caused a 41.4% relative reduction in nontiming-related errors and 50.8% relative reduction in potential nontiming adverse drug events.
11 One study showed 58% reduction in events in medical and surgical units but no reduction in the intensive care unit.
12 Another study showed that bar code–assisted medication systems reduced error rates in an adult intensive care unit by 56%.
The effectiveness of major new technologies, particularly bar code scanners, needs to be studied specifically in the context of anesthesia care in the operating room. The operating room is a complex environment, and introducing technologies that alter workflow may distract from other tasks. Interestingly, the study by Nanji and coworkers showed that labeling errors were the single most common error even when bar code scanners were available. Also, some anesthesia providers failed to use the bar code devices, suggesting that the devices were viewed as cumbersome or ineffective. The authors of the study recommended reducing the opportunity for workarounds such as removing manual sticker labels from the immediate workspace when bar code syringe labeling systems are available. However, we cannot assume that innovations, even those that “force function” and change behavior, actually improve safety. The effectiveness of bar code scanners needs to be assessed in the operating room before they are widely adopted.
Finally, Nanji and coworkers comment that “the perioperative areas are among the only remaining patient care areas that have not had rigorous assessment of medication events to guide proposed solutions.” This study has set the stage for future studies and effective interventions. Anesthesiologists have previously helped to develop a self-assessment checklist to improve medication safety in the operating room. These examples illustrate how anesthesiologists can and should lead change in the operating room. Leadership is essential to avoid situation where accreditation organizations, regulators, and others (e.g., those demanding cost-containment measures) impose interventions that have not been adequately assessed.
In summary, Nanji and coworkers are to be commended for performing the largest, observational study of anesthesia-related medication events available to date. Their results have confirmed a problem that anesthesiologists have suspected for years We must now promote change by doing what anesthesiologists do best: breaking down a tough problem into manageable pieces and then a building safer system for patients undergoing anesthesia.
Beverley A. Orser, M.D., Ph.D.; David U, B.Sc.Phm., M.Sc.Phm.; Michael R. Cohen, M.S., Sc.D. (hon.), D.P.S. (hon.)
-
From the Department of Anesthesia, University of Toronto, Toronto, Ontario, Canada (B.A.O.); Institute for Safe Medication Practices Canada, Toronto, Ontario, Canada (B.A.O., D.U.); and Institute for Safe Medication Practices, Horsham, Pennsylvania (M.R.C.).
-
Accepted for publication August 10, 2015.